Enhanced Lithotripsy System (ELS) Clinical Study
Current Study Overview
Avvio Medical is conducting a prospective, multi-center, single-arm clinical trial to evaluate the safety and efficacy of the Enhanced Lithotripsy System (ELS) for the fragmentation of urinary stones in the ureter (proximal, middle, and distal). This study represents a significant step in advancing stone treatment technology, offering a potential new solution for patients suffering from ureteral stones.
Procedure Animation
Avvio Lithotripsy vs Current Standard of Care
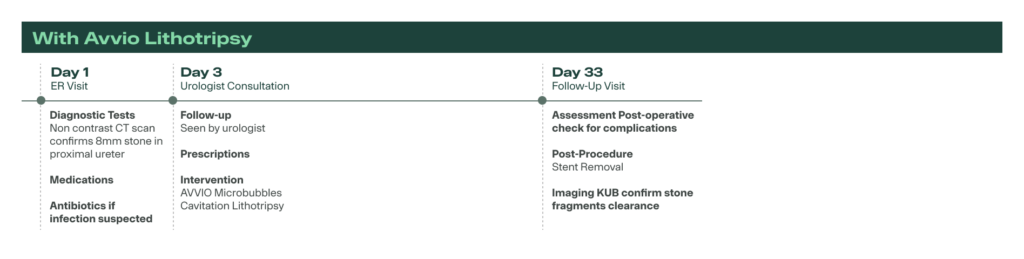
AVVIO accelerates stone care from weeks to days—avoiding delays, stents, and general anesthesia. Patients get faster, simpler treatment with fewer steps and less disruption.

Find a Participating Center
To find a participating site, please contact the site directly using the information provided. Don’t see a location close to you? Send us a note at info@avviomed.com — we’d be happy to help.
| Location | City, State | Phone | |
|---|---|---|---|
| Golden State Urology | Sacramento, CA | (916) 245-8888 | |
| Urology of San Antonio | San Antonio, TX | (210) 617-4116 | |
| Comprehensive Urologic Care | Lake Barrington, IL | (847) 382-5080 | |
| Freedman Urology | Las Vegas, NV | (702) 732-0282 x232 | drfreedman@freedmanurology.com |
CAUTION – Investigational device. Limited by Federal (or United States) law to investigational use. Avvio Medical, Inc. does not currently offer medical devices for sale or distribution and the company’s technology has not received market clearance status by the U.S. FDA or any other country’s regulatory authority.
MKT-006 Revision O, April 2025.