Acoustic Cavitation Lithotripsy

Low-pressure cavitation enhanced ultrasound. The world’s first system designed to enable an office based solution for kidney stone disease.
Avvio Medical aims to change the standard of care for the treatment of patients with urinary stone disease. Our novel office-based procedure enables shorter wait times without requiring fluoroscopy or general anesthesia, thereby reducing overall medical costs.
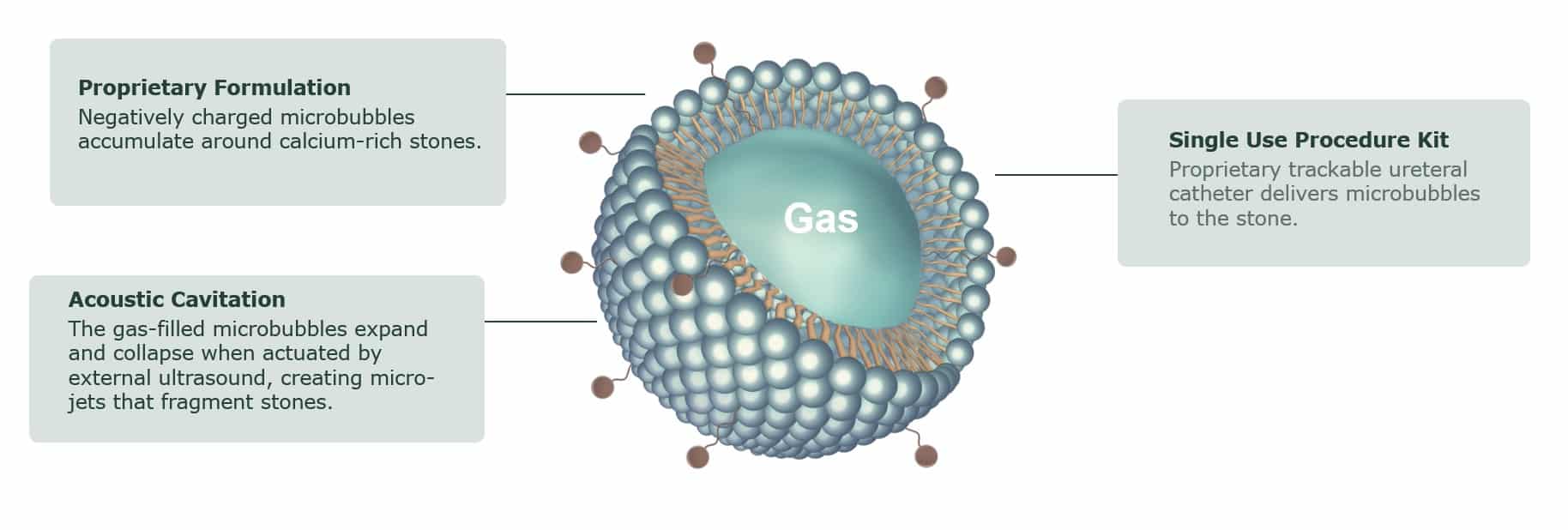
Our innovative technology employs low-pressure ultrasound to actuate calcium-seeking microbubbles, creating millions of micro-jets eroding the stone.*
Avvio Medical’s low-pressure ultrasound causes the Acoustic Enhancer microbubbles to expand and collapse, which helps break the ureteral stones.
What’s New
Applaud Medical Changes Name to Avvio Medical, Ushering in a New Era of Kidney Stone Treatment
San Francisco, California – Avvio Medical, Inc. (formerly Applaud Medical), a company dedicated to revolutionizing the treatment of urinary stones, today announced a bold step forward with a new name that reflects the Company’s vision for the future. "Avvio is more...
Favorable Clinical Data for Applaud Medical’s Novel Technology for Treating Urinary Stones Presented During the 2024 AUA Annual Meeting
San Francisco, California – Applaud Medical, an emerging leader in the treatment of urinary stones, today announced positive results from a prospective, multicenter study of the company’s BRIO Enhanced Lithotripsy System (ELS) for the treatment of obstructive ureteral...
Applaud Medical Appoints Paul Molloy as Chief Executive Officer and a Member of the Company’s Board of Directors
SAN FRANCISCO--Applaud Medical, an emerging leader in the treatment of urinary stone disease, announced today the appointment of industry veteran Paul Molloy (Molloy) as Chief Executive Officer (CEO). The company has also added Molloy to its Board of Directors....